Welcome to us
Our mission is to pioneer access for patients. In Slovenia, we develop, manufacture, and market effective, safe, and high-value generics and biosimilars. We are one of the leading partners in Slovenian healthcare.
Cornerstones of our success
Through our experience, expertise and quality we represent an important part of Sandoz. We dedicate a lot of resources to development, which is at the heart of what we do. We believe in the growing importance of scientific innovations that open pathways to advanced medicines and that improve access to treatment. We collaborate with scientific research organizations within Slovenia and abroad on the path to new discoveries.
Sandoz in Slovenia
Lek is the leading provider of biosimilars and one of the major providers of generics in Slovenia. Together with Sandoz d.d., we represent Sandoz in Slovenia. We are jointly building and protecting our reputation as a dynamic, ethical, and trustworthy pharmaceutical company.
We are delivering on:
- Sandoz's purpose – pioneering access for patients.
and
- Sandoz's long-term vision: to be the world's leading and most valued generics and biosimilars company.
Sandoz is increasing its investments in Slovenia. In addition to regular investments in the development, modernization, and expansion of production capacities, two Sandoz investments are underway:
- an investment in a high-tech biosimilars production center in Lendava, which is the largest single investment in Lek's history and one of the highest ever in the Slovenian economy;
and
- an investment to build a state-of-the-art biosimilars development center in Ljubljana; by 2026, Lek's Ljubljana site will become one of the key locations for the development of biosimilars in Sandoz.
Through continued investment, Sandoz is consolidating its presence in Slovenia, building a stimulating, competitive, and supportive work environment, and strengthening its role as a catalyst for the development of the pharmaceutical industry in Slovenia.
Lek within Sandoz
Lek is an important part of an international pharmaceutical company Sandoz.
We bring together more than 77 years of experience, knowledge, and achievements in the development and manufacture of quality pharmaceutical products from Sandoz sites around the world.
We are a Slovenian, European and global pharmaceutical company, people centered and future oriented.
We develop, manufacture and market effective, safe and high-value generics and biosimilars.
We are integrated in Novartis’ organizational structure, primarily in Global Drug Development, Novartis Technical Operations, Customer & Technology Solutions and generic division Sandoz. We take care of business functions and services for the wider region.
Our role within Sandoz:
- At Lek, we develop generic and biosimilar medicines, from the development of active pharmaceutical ingredients to finished products.
- We produce effective generics, including aseptic and solid products as well as anti-infectives.
- We lead and manage Sandoz's complex development projects.
- We package and distribute medicines.
- We market medicines on domestic and international markets.
Our success grows from knowledge
We invest heavily in the development, education and training of our employees. For decades, we have been actively co-creating the Slovenian research and development environment. We cooperate with Slovenian Universities and Centers of Excellence. We successfully link business and science.
Our capabilities and track record in the development of generics and biosimilars have made us a key part of Sandoz's global drug development program. Together, we are pushing the boundaries and increasing access to treatment for hard-to-treat diseases.
Lek's scientists and development teams regularly achieve outstanding scientific and commercial breakthroughs in the development of biosimilars and the manufacture of complex generics.
We are Sandoz's leading development center, carrying out half of all Sandoz development projects, and one of the key development sites for technologically demanding projects. We are proud to be at the forefront, introducing brand new products to global markets. Many development projects are the result of our knowledge, innovation, and experience.
We live quality at every step
Quality is embedded in our daily work, shown by trust and respect for employees, patients and business partner and the wider environment in which we operate. For many years, it has been recognized by successful audits of regulatory authorities from countries around the world.
High quality standards (as Good Manufacturing Practice, Good Laboratory Practice) are met in all work processes, in development and production of medicines as well as market supply with our highly qualified and experienced experts playing a key role.
We are a socially responsible company
We believe that business success and corporate responsibility are inextricably linked.
- We are working to increase access to treatment and raise awareness about the importance of health. Through research breakthroughs and innovation, we create new and more accessible treatment options for patients around the world.
- We are a responsible and ethical employer caring for the welfare of the wider community.
- We adopt a preventative approach, strive to make efficient use of natural resources and minimize our impact on the environment.
- We comply with the highest standards of ethical business conduct. Responsible business practice is the common guiding principle of our business strategy.
We share a common purpose
 Patients are in the center of everything we do.
Patients are in the center of everything we do.
We pursue Sandoz’ common purpose:
we are pioneering access for patients, which is reflected in our rich heritage and plans for the future.
and
Sandoz's vision – to be the world’s leading and most valued generics and biosimilars company: valued for how we run our business, but also for the positive impact we have on society.
The medicines we develop and manufacture are the result of decades of experience and expertise, which we nurture and connect with our values. Our investment in development is extensive and ongoing. Production follows strict international quality standards (like Good Manufacturing Practice - GMP).
Our values
Our culture is our collective identity. It is based on integrity and inclusiveness, with the aim that everyone can be the best version of themselves.
Our values help us achieve our mission and vision, select those who will work with us, develop talent, and reward achievements. They guide and connect us:
- Team up to break barriers. Work together to drive access.
- Be as ambitious as our purpose. Be bold to make change happen.
- Lead by example. Commit to making a difference.
- Open minds, open doors. Create new opportunities.
We provide a safe and inclusive working environment where all our associates feel accepted. This is essential for successful and committed associates who want to contribute to the achievement of common goals.
Our sites across Slovenia
The acquisition of knowledge and innovation are the driving force of all our sites. New investments enable us to expand our development and production capacities. Team mentality, supporting each other within a culture of inclusion and diversity help make everyone feel welcome and accepted at Lek. Our achievements are co-created by more than 3,700 associates at four sites across Slovenia: in Ljubljana, Lendava, Mengeš and Prevalje. We operate in development center and five production centers.
Lek incorporates four areas of operation::
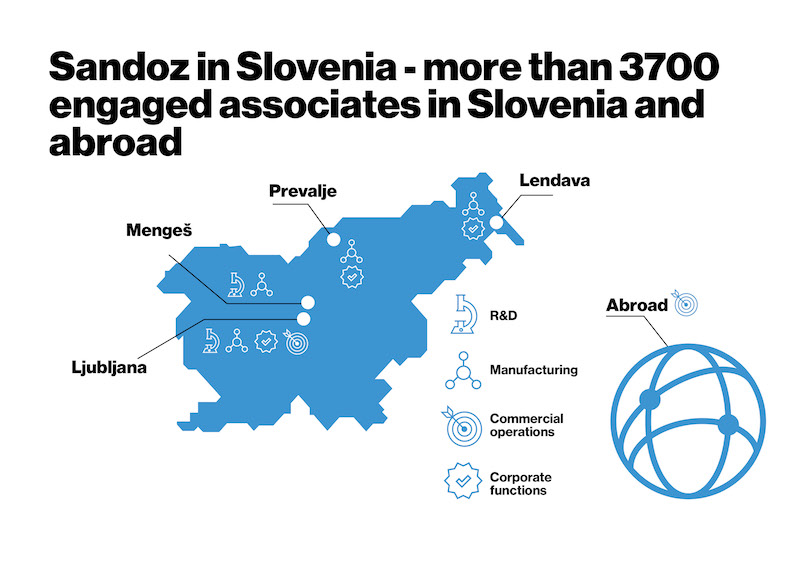
Ljubljana – In the center of Slovenia, at the heart of global development

In Ljubljana, more than 1,900 associates create a success story here at the headquarters of Lek d.d. and Sandoz d.d.
In addition to the production of aseptic products and other important Lek business and support functions, the site is also home to Sandoz's leading development center. In Slovenia, every year we develop on average 20 new medicines with a new drug substance or pharmaceutical form, or a medicine for a new market. Lek is thus making an important contribution to Sandoz's leadership in the generics and biosimilars market. An investment is also underway to build a state-of-the-art biosimilars development center; by 2026, Lek's Ljubljana site will have become one of the key sites for the development of biosimilars in Sandoz.
In the Aseptics unit, we produce approximately 70 different products and 500 finished products from more than 30 active ingredients. They are dispensed into sterile ampoules and vials, or are produced in the form of non-aseptic solutions, nasal sprays, and syrups for markets around the world.
We produce biosimilars and complex injectable products in the form of liquid vials and complex nasal sprays. We are also Sandoz's main hub for the transfer of new biosimilars, complex injectables, and nasal sprays from Sandoz’s development centers.

Mengeš
The Mengeš site is used for the production and packaging of Lek's probiotics, available in more than 50 markets worldwide. The production plant in Mengeš is part of Lek's Solids unit, which, together with the packaging facilities in Lendava, represents one of the most important production sites for solid dosage forms in Sandoz.
Lendava – In a corner of Slovenia, at the heart of progress

Our Lendava site is home to the production of anti-infectives and the packaging of finished pharmaceutical dosage forms, where more than 775 employees create stories of success. The market demand for potassium clavulanate, the active ingredient in the broad-spectrum antibiotic Amoxiclav, is growing, and the production capacity in Lendava is growing accordingly. Amoxiclav is our leading product.

Our site has a tradition of more than 30 years and we have demonstrated our commitment to the quality and safety of our active ingredients and their impact on the environment. Over the years, we have improved and adapted our processes to ensure that our active pharmaceutical ingredients are competitive in the generics markets.The environmental impact is also very important to us, and reducing emissions and energy consumption will be a top priority in the coming years.
The location is distinctly multicultural, as it employs associates of three nationalities: Slovenian, Hungarian and Croatian.
Being the largest solid pharmaceutical packaging plant in Sandoz, Solids is of strategic importance to Sandoz. We package nearly seven billion tablets and capsules a year, with more than 4,000 finished product codes, for patients in more than 120 countries around the world.
Our manufacturing facility produces generics from tablets and capsules sourced from several Sandoz factories around the world and from third-party suppliers. Lek's Solids unit also operates a manufacturing and packaging plant in Mengeš. With 28 packaging lines and more than 600 associates, together with our Mengeš plant we are one of Sandoz's most important and reliable suppliers of generics in solid dosage form.
At the same time, we are pursuing several important innovation initiatives in automation, digitization, and innovative packaging to meet our expectations for even greater competitiveness and operational excellence in the years to come. We are increasing access to drug treatments by finding new, innovative solutions that save on time, materials, and transport, and reduce our environmental footprint.
In Lendava, an investment in a high-tech biosimilars production center is under way. It is the biggest single investment in Lek's history and one of the largest ever in the Slovenian economy. The investment will strengthen the position of Lek and the Lendava site within Sandoz, as well as expand the range of products with high added value.
Prevalje

Production of broad-spectrum antibiotics at Prevalje ceased at the end of 2023. The analytical laboratory is being maintained for the purpose of providing stability and counter-indicator analyses of Prevalje products for an estimated four years and on a reduced scale.
Our associates are at the heart of everything we do
Sandoz is one of the largest employers in Slovenia. With more than 3,700 associates, we have been actively shaping the Slovenian, European, and global research and development landscape for decades. We are building a culture of open dialog, and we proudly and responsibly hold the title of the most reputable employer in Slovenia.
We respect and promote the cultural, ethnic, and gender diversity of our associates. We are creating an inclusive culture where all differences are valued, all practices are fair, and everyone feels respected. In this way, everyone can be the best version of themselves and fully embody our values. More than half of our senior positions are held by women. We are certified by the City of Ljubljana as an LGBT-friendly company. We value acceptance of everyone at every level.
Our Associates are our Future
We strongly encourage achievements of our employees. Every year, Novartis Slovenia bestows “Star” awards, recognizing those individuals whose work is particularly outstanding. Our operations are based on strong values, talent management is one of our priority areas, and with an unbossed culture we enable the associates to fully unleash their talents and creativity.
We actively focus on investing in the development and long-term support of young talented students and researchers. For many years we have been proactive in searching for talents and experts and carrying out internationally established HR projects.
ScienceBEAT
The annual ScienceBEAT event is a unique opportunity for young talented individuals to join a global network of professional excellence in science and entrepreneurship, and acts as a springboard for their careers. Selected participants gain valuable insights into the trends and challenges of the modern pharmaceutical industry and feel the pulse of a global pharmaceutical company that is a leader in the development and manufacture of pharmaceutical biosimilars and generics.
We work with educational institutions, student organizations, and career centers. At schools and colleges, as well as at the company, we regular make presentations on the trends in the pharmaceutical industry and share job opportunities for young professionals. Every year, we offer internships to science and engineering students, including foreign students. Many of them continue their career in our company after completing their education.
Through informal mentoring programs, the onboarding of new employees and the nature of our work, we foster collaboration and knowledge transfer between associates from different generations, disciplines, as well as different cultures (working in international project teams). Our older associates spontaneously assume the role of mentors transferring knowledge to younger colleagues through daily contact at work on scheduled tasks. Simultaneously, “reverse mentoring” is also provided in the form of continuous updating, especially IT skills, where younger associates pass on their skills to their older colleagues.
Innovation is our way of thinking and operating, it’s part of our daily effort towards continuous improvement of processes and products, for operational excellence and efficiency.
In the Company of the Most Reputable Employers
We have already been awarded the title of the most reputable employer on the Slovenian labor market five times in the Mojedelo.com survey, which confirms our efforts to build a supportive and high-quality working environment.
We have been fully certified as a Family-Friendly Company four times in a row. Lek has been participating in the Family-Friendly Company initiative since the very beginning of the project, demonstrating its commitment to creating a work environment that is considerate to employees and their families.
We understand that the only way our associates can grow in their jobs is by offering them new knowledge and skills. On average, Lek associates received 74.2 hours, or 9.3 days of training in 2022, with the highest numbers in Quality.
In Partnership with Professional Organizations
We are closely integrated into the Slovenian research and development environment, and our achievements are the result of joint efforts of the Slovenian scientific community. Companies and research institutions are our natural partners in the development of knowledge, basic skills, and applied solutions; together we create economic progress and provide a sustained growth of social well-being.
Our strategic partners in Slovenia are: The University of Ljubljana (Faculty of Pharmacy, Faculty of Chemistry and Chemical Technology, Biotechnical Faculty), The University of Maribor (Faculty of Chemistry and Chemical Engineering, Faculty of Mechanical Engineering, Faculty of Medicine), The University of Primorska (Faculty of Mathematics, Natural Sciences and Information Technologies), The Institute of Chemistry, and The National Institute of Biology.We also participate in several national and international programs with research institutions and other companies, where our role is focused on developing high-tech solutions for faster, more cost-effective, and safer production of medicines. We enable project consortium partners to test prototypes in an industrial environment and advise and guide other partners in the design and development of new technologies, products, and services to meet the needs of the biopharmaceutical industry.
The success of our development work is confirmed by numerous awards and acknowledgements for our experts for exceptional and sustainable scientific and business achievements both at the national and international level.
- Recipient of the gold and bronze national awards for innovative achievements 2023, awarded by the Chamber of Commerce and Industry of Slovenia.
- Recipient of the gold and silver national awards for innovative achievements 2022, awarded by the Chamber of Commerce and Industry of Slovenia.
Multiple recipients of the PHARM Connect Award for Excellence in Pharmaceutical Manufacturing, awarded at the largest business meeting of pharmaceutical and biotechnology companies from Central and Eastern Europe.
- Recipient of the Best of the Best 2022 Am Cham Slovenia best business practice award for the project Industry and University – Together Building a Society of Knowledge and Prosperity, implemented in collaboration with the Ljubljana University Incubator and the University of Ljubljana.
- Winner in the Innovation category of the AmCham Slovenia Best of the Best 2021 for the best business practice that brings innovation, motivation and collaboration to growth and progress in Slovenia;
- Recipient of the Responsible Care Program® certificate – RCP for Lek’s continuous efforts in health and safety, environmental protection and transparent reporting on its activities in this field;
- Golden award for the best innovation at the national level 2020, presented by the Chamber of Commerce;
- The associates of Lek are the recipients of the Prometheus in Science award for communication excellence;
- Two Golden National Innovation Awards 2019 bestowed by the Slovenian Chamber of Commerce and Industry – over the past 17 years since the first national innovation awards were presented, we have received 11 golden awards;
- Golden National Innovation Award 2017 bestowed by the Slovenian Chamber of Commerce;
- Pregl Award 2016 – Zdenko Časar, PhD, Development Center Slovenia;
- Puh Award 2014 – a team of researchers from Biopharmaceuticals Mengeš in cooperation with their colleagues from the National Institute of Chemistry;
- Acknowledgement to Biopharmaceuticals Mengeš for a long-term successful cooperation in research and professional work (Biotechnical Faculty of the University of Ljubljana);
- Plaque for longstanding cooperation in practical training of students (Faculty of Chemistry and Chemical Technology of the University of Ljubljana);
- Award for innovative and efficient human resource practice in Slovenia within the Golden Thread project Golden Practice 2020 for the Young Advisory Board project


