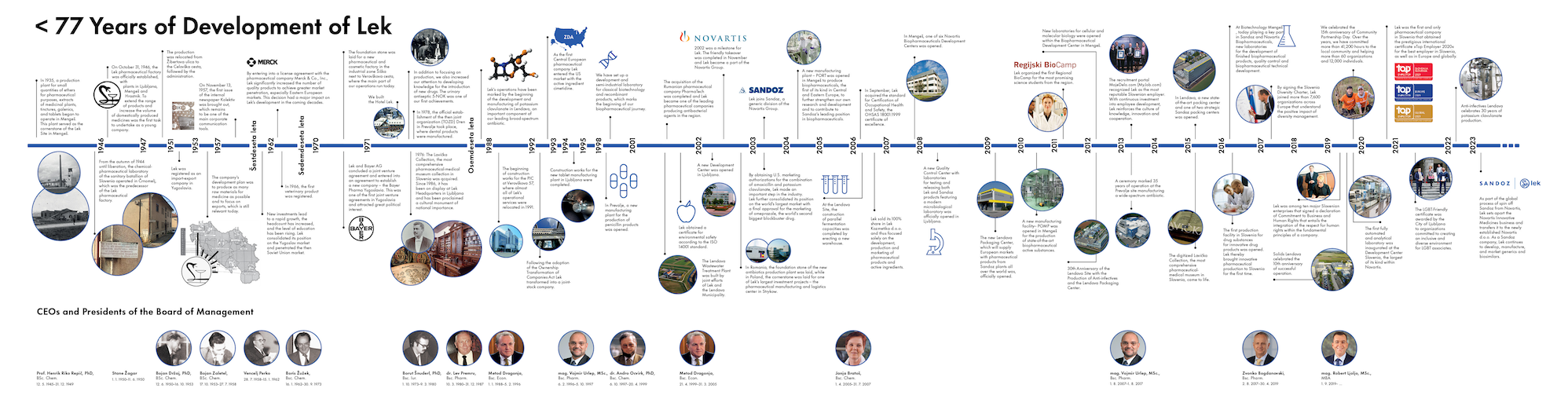
History
We are the first and oldest modern pharmaceutical company in Slovenia. From the very beginning, our operations have been characterized by dynamic development and a predisposition towards internationalization. Constant and intensive growth, characteristic of Lek’s entire history, has recently become an especially noticeable factor.
More than 77 Years of Development of Lek
1946
The Lek medicinal products factory is officially founded. As a young company, the first task is to expand the product portfolio and to increase the number of in-house manufactured drugs.
increase the number of in-house manufactured drugs.
1965
New investments introduce a period of rapid growth, the number of employees increases and their average level of education rises.
The eighties
Lek’s operations are marked by the beginning of the development and manufacturing of potassium clavulanate, an important component of our leading product Amoksiklav®.
1992
Following the adoption of the Transformation of Company Ownership Act at the end of 1992, in June 1994 Lek becomes the first Slovenian joint-stock company with known private owners. In June 1992, Lek’s class C shares are first quoted on the Ljubljana stock market, followed by class A shares in August 1996 and, after dematerialization, class B shares in 1999.
1993
As the first Central European pharmaceutical company Lek entered the US market with the active ingredient cimetidine.
1998
We establish a research and semi-industrial laboratory for classical biotechnology and recombinant products. In Prevalje we open a new manufacturing plant for the production of penicillin products.
2000
In accordance with our strategic orientation to the US generics market we obtain the registration of enalapril, one of the highest-selling drugs on that market. We lay the cornerstone of the new Development Center, one of our major investment projects in this period. We open a new business center for south-eastern Europe in Skopje (Macedonia) and a representative office in Riga (Latvia).
 2001
2001
We complete the acquisition of the Romanian pharmaceutical company Pharma Tech and become one of the leading manufacturers of antibiotics in the region. This is followed by the acquisition of Argon S.A. in Lodz, Poland, through which Lek strengthens its position on the Polish market and in the cardiovascular and OTC segments. On the American market we found Lek Pharmaceuticals, Inc. and become a distributor of medicines in that market. We open a representative office in China and obtain a certificate for environmental safety according to the ISO 14001 standard.
 2002
2002
2002 is a landmark year for Lek. The friendly takeover through which Lek becomes a part of the Novartis Group is completed in November
On the American market we obtain marketing approvals from the regulatory agencies for the sale of bromocryptine, a drug for treating Parkinson’s disease, and lisinopril, a drug for treating high blood pressure. In Ljubljana we open the new Lek Development Center, which enables the closer cooperation of Lek’s research units with research activities at scientific institutes and universities at home and abroad. In Romania we lay the cornerstone of the new manufacturing plant for the production of safe and high-quality antibiotics, and in Poland the cornerstone for one of Lek’s largest investment projects – the pharmaceutical manufacturing and logistics center in Strykow.
 2003
2003
The obtaining of a marketing approval for launching co-amoxiclav on the US market in 2003 is a major step in operations. We further strengthen our position on the world’s largest market by obtaining a marketing approval for omeprazole, the second-highest selling pharmaceutical product in the world. We also successfully defend our European patent for the synthesis of omeprazole.
We receive Innovator of the Year award in 2002 for the project “Filgrastim – acquiring a recombinant protein through innovative solutions”.
After recording successful results Lek takes first place among Slovene companies, overtakes regional competitors in terms of growth and achieves the highest sales of comparable pharmaceutical companies in Central and Eastern Europe.
In May 2003 several national brands in the framework of Novartis Generics are formed into a strong global brand: Sandoz.
 2004
2004
We open a new biopharmaceuticals plant in Mengeš, the first of its kind in Central and Eastern Europe. With it we strengthen our in-house research and development and contribute to Sandoz’s achieving a leading role in the field of biopharmaceuticals.
The new plants in Romania and Poland begin operating, which will manufacture drugs for the growing needs of the European markets and strengthen Novartis’ commitment to the markets of the expanded European Union.
Sandoz begins marketing pravastatin, a drug for lowering cholesterol, in Great Britain, Germany, the Netherlands and Denmark. The drug is the fruit of Lek’s in-house knowledge and the successful linking of Lek’s development, manufacturing and patent protection divisions with the opportunities offered by Sandoz’s global network.
Together with the Faculty of Medicine in Ljubljana we enable the publishing of the electronic edition of the Slovene Medical Dictionary, and together with professional associates we enable the publishing of a new edition of the publication Arterial Hypertension.
 2006
2006
In September we laid the cornerstone for the Lek Biopharmaceuticals Development Center in Mengeš, where we will develop and produce biopharmaceuticals for Sandoz’s global markets. In Ljubljana we upgraded a new line for the technically demanding technology of statin tablets. We started a project of expanding production, which envisages an increase in annual production capacities to 5 billion tablets. In Lendava we completed a project of constructing parallel fermentation capacities by erecting a new warehouse.
We sold our 100% share in Lek Kozmetika d.o.o. and thus focused solely on the development, production and marketing of pharmaceutical products and active ingredients.
In the field of occupational health and safety in September we acquired the OHSAS 18001:1999 certificate of excellence.
In 2006 we initiated a difficult project, as part of which we organized the field of personal data security in accordance with legislation, and won an award for good practice in personal data security.
 2007
2007
In October we opened the new Biopharmaceuticals Development Center.
In Ljubljana we built a new quality assurance center with laboratories for testing and releasing Lek and Sandoz products, as well as a modern microbiology laboratory.
At the end of the year we completed our project of restructuring and optimization of processes in Supply, which became the Sandoz supply center for the markets of CEE, SEE, CIS and Slovenia.
In Lendava we began construction of a regional packaging center.
On May 31 a US District Court in New York found that Lek’s product Omeprazole, a generic version of Prilosec (omeprazole) did not infringe originator Swedish-American pharmaceutical company AstraZeneca’s patents.
We were among the first companies in Slovenia to receive a “Family-Friendly Company” certificate. At the end of the year we completed the construction of a new day-care center in Ljubljana.
 2008
2008
A new quality control center with laboratories for testing and releasing both Lek and Sandoz products featuring a modern microbiology laboratory was opened in Ljubljana. At the same location, we have established a new dispensary and Werum weighing, which is the most complex one in the Sandoz group.
At the end of September, we completed the expansion of sterile lyophilized forms (lyophilizes) at the sterile product manufacturing plant. We have completed the expansion of the production of sterile lyophilized form.
We introduced a new medication containing perindopril for treating high blood pressure to the Slovenian market.
We received the Slovenia award TOP 10 - 2008 for systematical investments in employee training. We obtained the "Responsible Care" certificate for our Responsible Behavior Program.
Sandoz received European Commission’s approval for our third biosimilar medicine – recombinant protein filgrastim. By doing so, Sandoz further reinforced its position as a pioneer in the field of biosimilars. Filgrastim is a significant part of the development results of our experts.
 2009
2009
At the end of September we officially opened the new Lendava Packaging Center, which supplies European markets with finished pharmaceutical products from Sandoz plants all over the world. In five years, the investments in Slovenia amounted to 750 million EUR.
Lek won three Superbrand awards for our brands Lek®, Lekadol®, and Persen®, which ranked us among the strongest domestic and foreign brands on the Slovenian market.
For the fifth consecutive year in a row, we held the annual Community Partnership Day with more than 180 Sandoz and Novartis associates in Slovenia joining thousands of other Novartis associates worldwide.
The Faculty of Medicine of the University of Ljubljana, in cooperation with Lek, a Sandoz company, has published an electronic version of the 4th edition of the Slovenian Medical e-Dictionary.
 2010
2010
In Mengeš, we opened a new facility for the production of state-of-the-art biopharmaceutical active pharmaceutical ingredients, which has enabled us to use this site for the production epoetin alfa drug substance. This new facility will produce modified proteins – the next generation of proteins featuring enhanced biological medicinal products.
This further strengthened the role of Lek within the Sandoz biopharmaceutical development and production network. We have proved that the Slovenian expertise in recombinant technology can compete with the world’s top players.
We were the first company in Slovenia to offer generic drug substance rosuvastatin* for lowering cholesterol. The medicine has been produced and developed in Slovenia.
Lek received a full Family Friendly Company certificate, confirming our commitment to helping its associates balance their professional and private lives.
 2011
2011
In one year, Lendava Packaging Center has packed over billion tablets and became a key Sandoz location. With this we have entered the Sandoz league of large productions and key locations.
Lek hosted the first Regional BioCamp in the Alpe-Adria region with distinguished experts from Sandoz and Novartis meeting top undergraduate and graduate science students.
The third scientific conference Sandoz Research and Development (R&D) Day was held and hosted in Bled for the first time.
On Community Partnership Day, we hosted a record number of blood donors.
 2012
2012
We celebrated 30th years anniversary of operation in Lendava site. In this time we have become a major employer in the region. We also celebrated the 20th anniversary of potassium clavulanate production – the key components of the broad-spectrum antibiotic and one of the leading and best-selling Sandoz products.
In Mengeš, we opened new laboratories for cellular and molecular biology further strengthening our support for Sandoz Biopharmaceuticals in maintaining the leading role in the development of biosimilars.
The management board of the Managers Association selected Vojmir Urlep, President of the Board of Management of Lek, a Sandoz company, as Manager of the Year 2012.
We received our tenth TOP 10 award for systematical investments in employee training.
As part of the Asthma & Sport program, we organized the 5th Lek cycling marathon for raising awareness of pulmonary diseases and the importance of taking care for our lungs.
We topped off our environmentally and socially responsible operations by adding all our development and manufacturing locations in Slovenia to the EMAS scheme – a voluntary environmental management instrument of the European Union.
 2013
2013
Vojmir Urlep, President of the Board of Management of Lek, received the award for outstanding business achievements from the Chamber of Commerce and Industry of Slovenia.
35 years of operation at the Prevalje site was marked with a ceremony. Over these past years, this once small pharmaceutical plant has grown and developed into an important and modern part of Sandoz. The production volumes of its key product – broad-spectrum antibiotic – have been continuously growing; therefore new jobs keep being created.
Sandoz was the first to market generic mometasone nasal spray in Slovenia for the treatment of seasonal or perennial allergy rhinitis and symptomatic treatment of nasal polyps.
We have received the Invest Slovenia FDI Award as one of the most successful foreign direct investment in the country.
The recruitment portal MojeDelo.com recognized and awarded Lek, a Sandoz company, as the most reputable Slovenian employer.
On the occasion of the 60th anniversary of the Slovenian Association of Friends of Youth and as part of our long-term partnership we helped them mark their remarkable anniversary and celebrate 15 years of the Wink at the Sun campaign – organized to enable free holidays for children from socially disadvantaged backgrounds.
 2014
2014
A team of scientists at the Mengeš Biopharmaceuticals and the National Institute of Chemistry Ljubljana received the Puh Award for developing processes for the production of biosimilars filgrastim and pegfilgrastim and innovative methods to transfer them into production.
We maintained our role as a stable employer in the central Slovenian region, Carinthia, and Prekmurje and increased the number of employees by 5%.
We invested almost 3 million Euros in environmental protection.
For the tenth time in a row, the Community Partnership Day was organized in Slovenia. Throughout the past ten years, Novartis associates in Slovenia have carried out 23,000 hours of voluntary work and helped more than 10,000 people and 40 organizations.
 2015
2015
The US Food and Drug Administration (FDA) approved Sandoz biosimilar filgrastim for treating patients with increased risk for neutropenia. Neutropenia occurs often following cancer treatments, as well as advanced HIV infections. Actively involved in the initial stage of the development of recombinant filgrastim was a team of expert scientists from Lek, a Sandoz company, and the National Institute of Chemistry in Ljubljana. The launch of the first biosimilar filgrastim in US has paved the way for greater access to high-quality biologics. Sandoz has become the first company to introduce a biosimilar in the US – leading the way in the US just as it did in Europe nearly 10 years ago.
At the Lendava Packaging center, we opened a new logistic system, which is the largest single investment made by Novartis in Slovenia so far, and created new jobs. The Lendava Packaging center features environmentally-friendly and innovative technology in accordance with Novartis environmental standards.
At the fifth Regional BioCamp we spoke about new strategies for the treatment of autoimmune diseases.
The most comprehensive pharmaceutical-medical museum collection in Slovenia, the Lavička Collection, came to life in digital form.
* The medicine is available in Slovenia under the name Zarzio and is registered via a centralized procedure.
2016

2016 was a jubilee year for us. We celebrated 20 years of Novartis and the venerable 70 years of Lek. We have enriched this important year with various projects and activities for the development of knowledge and innovation, with new investments in the expansion of development and production capacities and environmental technology, by promoting a healthy lifestyle and strengthening our corporate responsibility. In this way, we live and realize our values with even greater pride and celebrate our common successes.
2017
We signed the Slovenian Diversity Charter, thus further strengthening our activities in promoting a culture of diversity and inclusion in all parts of the company.
The Volunteer Industrial Fire Brigade Lek (PIGD), which brings together more than 180 volunteer members employed by Lek or other companies operating for Lek, celebrated its 70th anniversary.
We chose a strategic buyer for Hotel Lek in Kranjska Gora.
2018
Our associates received the highest Novartis scientific award. Assoc. prof. Zdenko Časar was the first Slovenian scientist to receive Novartis top scientific honour, the Distinguished Scientist Award.
Novartis CEO Vas Narasimhan, visited Slovenia.
We participated in the second Summer School for secondary school students organized by the Ljubljana University Career Center and offered the youngsters an insight into the world of the pharmaceutical industry.
In the five years of the Th!Nk Novartis application, intended to encourage employees to generate new ideas, the associates in Slovenia submitted more than 5,500 proposals. In fact, it was in Slovenia where the application had its pilot run.
2019
Our researchers received the Prometheus in Science Award for Communication Excellence, and the title »Star« of the Slovenian Science Festival.
The Development Center Slovenia team received the highest annual Sandoz Award in the category Operational Excellence.
We organized the 15th Community Partnership Day. Over 15 years we helped more than 60 different organizations and 12,000 people as part of the Community Partnership Day initiative.
We signed a declaration of Commitment to Business and Human Rights, together with nine other major Slovenian enterprises.
Together with our development partners, the Faculty of Mechanical Engineering and Faculty of Pharmacy, we received the TARAS award for the best collaborative project in industry for the project Artificial Stomach.
We celebrated the 10th anniversary of successful operation of Solids Lendava that over ten years evolved from a start-up unit into a global strategic Novartis unit for the final stage of drug production.
In the Development Center Slovenia, we opened new development laboratories that enable also the development of solid and sterile dosage forms for cancer treatment.
We received two golden national awards for innovation – for the dietary supplement LinComplexTM and for the continuous and integrated process for the purification of biopharmaceuticals
We organized the first Novartis career recruitment event in Slovenia visited by more than 130 participants.
Our scientists from the Development Center Slovenia and Biologics Technical Development Mengeš received nine Sandoz awards for research and development.
For the second time, we received the prestigious title of the most reputable employer bestowed to Slovenian employers by the recruitment portal MojeDelo.com
At the Development Center Slovenia, we opened the first fully automated analytical laboratory.
2020
For the twelfth time we were among the recipients of the Responsible Care Program certificate®.
At the PHARM Connect congress, we received the Manufacturing Excellence Awards for the implementation of data science in generic medicine development projects.
Novartis provided Slovenia USD 500,000 in grants to support the fight against the COVID-19 pandemic. The funds were donated to the Slovenian Red Cross and the Slovenian Association of Friends of Youth.
Novartis donated of a substantial amount of hydroxychloroquine to treat hospitalized COVID-19 patients in Slovenia.
President of the Republic of Slovenia Borut Pahor visited the Novartis Slovenia site in Mengeš that at the onset of the COVID-19 pandemic started manufacturing disinfectant to be donated to local communities.
We donated computers to elementary schools in Lendava, Ljubljana, Domžale and Prevalje, to help children follow distance learning during the COVID-19 pandemic.
Our scientists were once again honored with Novartis awards for outstanding achievements in research and development.
We took part at the Innovation Day hackaton, organized together with the European Institute of Innovation and Technology (EIT Health) and Ljubljana University Incubator.
Scientists from the Development Center Slovenia and Biologics Technical Development Mengeš received 17 Sandoz awards for research and development.
We received the highest national recognition for innovative achievements, presented by the Chamber of Commerce and Industry of Slovenia, for the development of a new generic medicine with tacrolimus in the form of prolonged-release capsules, intended for the treatment of organ transplant patients.
Novartis in Slovenia was the first and only pharmaceutical company in Slovenia to obtain the prestigious international certificate »Top Employer 2021« for the best employer in Slovenia, as well as in Europe and globally.
2021
We organized the sixth Novartis career breakfast, which was attended by over 300 experts in natural and other sciences. The event was held online for the first time.
We received an LGBT Friendly Company certificate awarded by the Ljubljana City Municipality.
We received the Golden Practice Award 2020 for the Young Advisory Board (YAB) initiative promoting innovative approaches to the growth and development of the associates.
Our project Vision that introduces mixed reality technology, won the Innovation category of the Best of the Best 2021 project, with which AmCham Slovenia recognizes best business practices.
At our Ljubljana and Mengeš sites, we experimentally set up charging stations for personal electric and hybrid vehicles of employees and partners. We also introduced the first electric delivery vehicle in the cold chain in Slovenia and one of the few in the world for the distribution of analytical samples in the pharmaceutical industry, which runs between the two development and production sites.
Novartis in Slovenia was recognized as the most reputable employer 2020 in Slovenia. We received this title for the second consecutive year, for the third time in total.
We marked 25 years of Novartis and 75 years of Lek.
We celebrated the15th anniversary of the first approved biosimilar in the world. It was developed and manufactured by Sandoz.
2022
We have been awarded the Disability Employment Good Practice Award 2021 and the Disability-Friendly Company Award for the important steps we have taken in the field of disability employment.
At Sandoz R&D Days, Lek experts received a total of ten scientific awards for their outstanding contribution to research and development and to increasing access to high-quality medicines. The awards are a recognition that our researchers are consolidating their position as leading experts in the development of biologics, biosimilars, and generics at Sandoz.
Anti-infectives Lendava celebrated 30 years of production of potassium clavulanate, the active ingredient of Sandoz's best-selling broad-spectrum antibiotic, Amoxiclav.
Bioproduction Mengeš was awarded the title of Factory of the Year 2022. It impressed the expert panel with the high level of automation, digitization, and use of data science, as well as its culture of empowerment, innovation, and curiosity.
For the third time in a row, the fourth time in total, we have been voted Slovenia's most reputable employer.In the research, candidates in the labor market selected Lek as the absolute winner and the winner in all individual categories.
Our locations across Slovenia have become dementia-friendly hotspots.
We became a silver sponsor of the Federation of Sports for the Disabled – Slovenian Paralympic Committee (ZSIS – SPK).As part of this collaboration, we are working to improve the involvement of people with various forms of disability, both inside and outside the company.
2023
In the presence of Prime Minister Robert Golob and President of the Sandoz Board of Management, Richard Saynor, we signed a Memorandum of Understanding for the construction of a high-tech center for the production of biosimilars in Lendava. The planned investment is the biggest single investment in Lek's history and one of the largest in the Slovenian economy.
As part of the global process of spin-off Sandoz from Novartis, Lek d.d. sets apart the Novartis Innovative Medicines business and transfers it to the newly established Novartis d.o.o.
As part of the Sandoz Group, Lek continues to develop, manufacture and market generic and biosimilar medicines.